Contact
Contact
Zhongguancun North First Street 2
Beijing, 100190, PR China
Institute of Chemistry,CAS
Group of Photocatalysis and Environmental Photochemistry
Tel:010-82615942
Fax:010-82617315
Email:jczhao@iccas.ac.cn
 Introduction
Introduction
The environmental problems caused by organic pollutants are becoming more and more serious all over the world. The interests of our group are focused on the novel approaches for the photodegradation of environmental pollutants, photochemical cycling of iron species and the relative environmental photochemical processes, novel photochemical approaches for the detection of trace hazardous compounds, and photocatalytic selective oxidation of organic compounds. We have published over 400 papers in well-known international journals, such as J. Am. Chem. Soc., Environ. Sci. Technol., Angew. Chem. Int. Ed., and have more than 30000 citations by others. We have been awarded with several prizes such as the Second Grade National Prize of Natural Science of China , the Japanese Photochemistry Association Lectureship Award for Asian and Oceanian Photochemist (2010) and the Award of Excellent Young Scientists of Chinese Academy of Sciences- Bayer. Team members include 9 professors, 1associate professors, 6 postdoctors and 48 graduate students.
 Latest Research Progress
Latest Research Progress
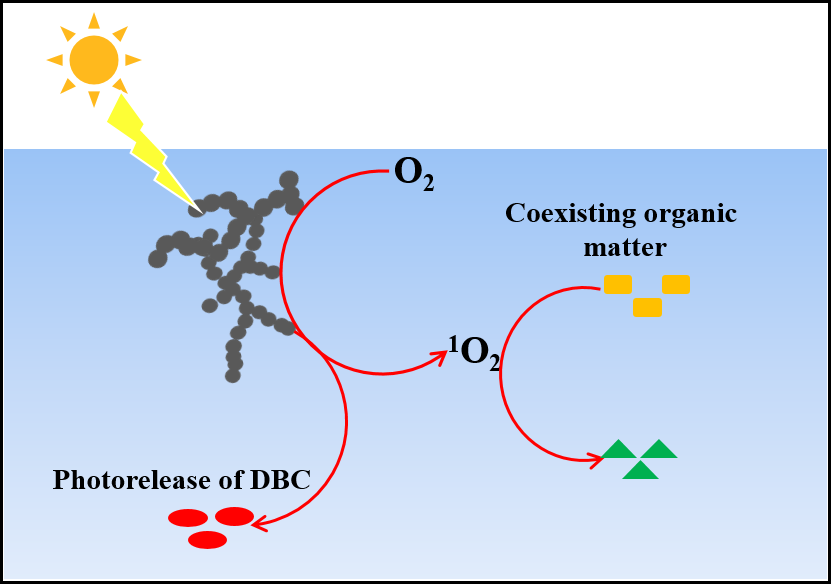
Photochemical Aging of Soot in the Aqueous Phase: Release of Dissolved Black Carbon and the Formation of 1O2
The photochemical aging of soot in the aqueous phase could have an important influence on water environments such as fog water and wet aerosols in the atmosphere, as well as lakes and oceans. In this study, we systematically investigated the photochemistry of soot in the aqueous phase. Soot releases dissolved black carbon into the aqueous phase during photoreactions, which is attributed to the phototransformation of the nonpolar unsaturated C-H species in soot to polar carbonyl-containing species. More importantly, we found that soot suspensions, particularly those of the dissolved part of soot, were effective photosensitizers for the generation of singlet oxygen (1O2). The 1O2 apparent quantum yield of the dissolved part reached 33 ± 2% under 377 nm irradiation, which is an order of magnitude higher than those of most types of well-studied dissolved organic matter in water. As a result, soot could impact the environmental fate of coexisting organic contaminants, such as the photodegradation of bisphenol A. This study will not only give insight into the photochemistry of soot in the liquid phase but also reveal the significant implications of soot photoaging in the aqueous phase by the release and degradation of organic matter. (Environmental Science & Technology, 2019, DOI:10.1021/acs.est.9b02773)
 Research Progress
Research Progress
内容
Most solar-energy conversion applications are based on trapping and transferring photoinduced electrons on oxide semiconductor nanoparticles, such as titanium dioxide, and broad UV-vis absorption (400~800 nm) and monotonic IR absorption (1100~3000 cm−1) signals have long been considered signatures of the electron-trapping state on titanium dioxide nanoparticles. Here we show that, under proton-free conditions and using iodide ions in acetonitrile as the hole scavenger, the intrinsic electron-trapping feature of titanium dioxide nanoparticles does not exhibit the characteristic UV-vis absorption and infrared absorption signatures. Further electron spin resonance studies identify the proton-free electron-trapping state as the lattice octahedral Ti6c3+ species, differing from the traditional protonparticipating surface tetrahedral Ti4c3+ species. Synchronized radiation ultraviolet photoelectron spectroscopy results also show that the internal electron-trapping state without protons has a larger Ti3d binding energy (1.8 eV) than the blue electron-trapping state (1.3 eV) that forms when protons participate and thus shows different electron transfer abilities. (Communications Chemistry. 2019, 88. DOI: s42004-019-0191-7)