Research Progress
Research Progress
内容
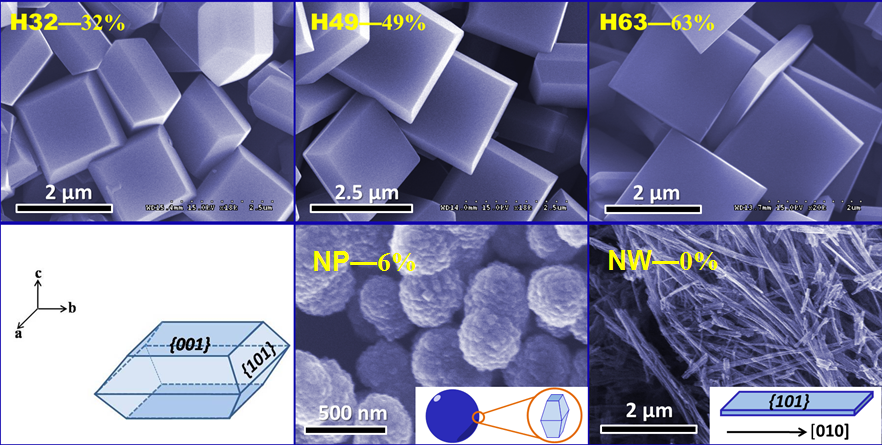
The photocatalytic oxidation reaction in the aqueous TiO2 system has aroused intensive research interests owing to its significance in the efficient elimination of environmental contaminants and synthesis of fine organic chemicals. During the reaction process, both water and dioxygen will take part in the reaction. Depending on the reaction pathways, the dioxygen molecule will either become the thoroughly reduced product, namely water, or be incorporated into the organic substrate as a functional group. Water, acting as both solvent and reactant, is either oxidized by photogenerated holes, producing hydroxyl radicals, or reacts with organic radicals, producing hydroxylation intermediates. Consequently, in the anatase-photocatalyzed oxidation reaction, the oxygen atoms introduced in the substrates originate from both water and dioxygen. In the present study, a series of anatase titania crystals were synthesized in which the percentage of exposed {001} facet varied over a wide range(0%, 6%, 32%, 49%, 63%). Photocatalytic oxidation of terephthalic acid was chosen as the model reaction. We found that {101} facet exhibits higher photocatalytic activity than {001} facet, but the contribution ratio of dioxygen to water in the hydroxylation reaction is higher on the {001} facet than on the {101} facet; during the successive oxidation process, more dioxygen was consumed and incorporated into the substrate on the {001} facet than on the {101} facet upon converting an equal amount of substrate. Based on these experimental facts, it is believed that the pathways, by which dioxygen participates in the reactions, are different on {001} and {101} facets.